Location
CoLab, COM 100
Start Date
1-5-2025 11:00 AM
Document Type
Poster
Description
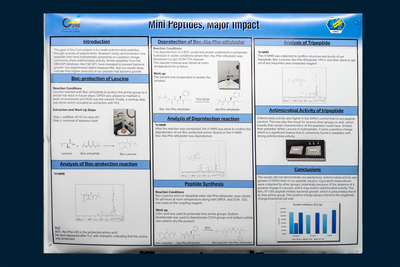
The intention of this CURE project is to develop antimicrobial peptides that are made up of 2-3 amino acids. The tripeptide, BOC-Leu-Ala-Phe-OEt, will be the specific sequence attempted by this group. Research today demonstrates how peptides that have hydrophobic properties or a positive charge commonly reveal some antimicrobial activity. A series of experiments must be conducted for the peptides to be created. To ensure that the right peptide bond is made, protecting groups will be utilized to avoid side-reactions between free amino and carboxylic acid groups. The amine group in Leucine will be protected by a BOC- group so the carboxylic acid can react. Thin layer chromatography and NMR will be used to ensure that the correct product has formed. An ethyl ester will be used as the protecting group for the peptide, BOC-Ala-Phe- OEt, to protect the carboxylic acid group. However, The BOC-group will first be detached, allowing only the amine group to react. The final task of this series of experiments is for the peptide bond to be formed with EDC which is a coupling reagent. Once the amino acids are protected and the final peptide is synthesized, ¹H NMR will be used to confirm the structure and purity.
Synthesis and Testing of Antibacterial Peptides
CoLab, COM 100
The intention of this CURE project is to develop antimicrobial peptides that are made up of 2-3 amino acids. The tripeptide, BOC-Leu-Ala-Phe-OEt, will be the specific sequence attempted by this group. Research today demonstrates how peptides that have hydrophobic properties or a positive charge commonly reveal some antimicrobial activity. A series of experiments must be conducted for the peptides to be created. To ensure that the right peptide bond is made, protecting groups will be utilized to avoid side-reactions between free amino and carboxylic acid groups. The amine group in Leucine will be protected by a BOC- group so the carboxylic acid can react. Thin layer chromatography and NMR will be used to ensure that the correct product has formed. An ethyl ester will be used as the protecting group for the peptide, BOC-Ala-Phe- OEt, to protect the carboxylic acid group. However, The BOC-group will first be detached, allowing only the amine group to react. The final task of this series of experiments is for the peptide bond to be formed with EDC which is a coupling reagent. Once the amino acids are protected and the final peptide is synthesized, ¹H NMR will be used to confirm the structure and purity.

Comments
The faculty mentor for this project was Meagan Weldele, Chemistry.